(Montagu, 1813) from Sado estuary: effect of body size on accumulationsUMMarY: concentrations of Fe, Zn, cu, Pb and cd were measured in four surface sediments, whole worm tissues and faeces collected along Águas de Moura channel in sado estuary. six wet-weight worm classes were used to analyze the influence of weight. the metal concentration in colonized sediments is high for Fe and cu, moderate for Pb and Zn, and low for cd. the analyses of whole worm tissues show that Zn, cd and cu are accumulated. considering the elevated sediment concentrations to which this species is exposed, the high levels of Zn and cu suggest sequestration and cu adaptation. However, the low Fe concentration indicates that this metal is not readily available. the similar Pb behaviour also suggests low availability or Fe interference. the high correlation in the sediment between Pb and Fe reinforces this suggestion. the results obtained show that Pb, Zn and Fe are the most important metals in the different weight classes. the overall results of this study show: (1) that this worm is able to adapt physiologically to elevated levels of metals; and (2) that the weight of the worms needs to be taken into account in environmental management programmes.
IntroDUctIonestuaries are areas of high productivity, crucial in the life history of many fish, invertebrates and birds. the sustainability of estuarine biodiversity is vitalto the ecological health of coastal regions. estuaries also rank among the most anthropogenic ecosystems on earth and are subjected to intensive environmental pressures. In particular, sediments can act as a sink and cycling centre for metallic contaminants, and therefore can be a potential source for metal bioac- cumulation by marine deposit and suspension feed- ing invertebrates, which may have adverse effects at complex levels of biological organization (lee et al., 2000). one major concern with the chemicals associated with sediments is that many commercial species, and their preys, are particularly vulnerable to toxic compounds given their close contact with sediment particles and interstitial water for extended periods of their life cycle. this provides a pathway for these chemicals to be transferred directly from sediments to organisms (Wright and Mason, 1999). Determining the ecological significance of trace metal contamination in sedimentary environments is difficult. Uptake and effects of sediment-associated contaminants are largely a function of bioavailabil- ity, which is strongly influenced by a set of physical, chemical and biological factors in the sediments. While most metals are naturally present in the aquatic environment, it is their presence at elevated concentrations that is a potential threat to aquatic life (rainbow et al., 1990).It is important, therefore, to determine whether ecologically keystone species of our estuaries are at risk due to toxic contaminants or whether different local populations are tolerant to bioavailable levels of toxicants that are potentially lethal elsewhere. In Sado estuary M. sanguinea is a herbivorous and sur- face detritivorous feeder with moderate or discreet surface mobility. It inhabits the intertidal mudflats of estuaries and coastal zones. It is broadly distributed in sado estuary (Portugal) wetlands, where it lives in deep borrows, and is particularly abundant in oldoyster production areas. It is among the key species in sado estuary, and functions as a major constitu- ent of the benthic biomass of mudflats as well as an important food item for crustaceans, fishes and waders (castro, 1993). M. sanguinea is also com- monly used as fresh bait and harvesting it is one of the most important socio-economic resources for local fishermen.the purpose of this study is to evaluate the influ- ence of weight on the bioaccumulation of sediment- bound Fe, Zn, cu, Pb and cd in M. sanguinea in Águas de Moura channel, located in central sado estuary.
MaterIal anD MetHoDsStudy areasado estuary is located on the southwest coast of Portugal (37º25'-38º40'n, 0.07º40'-0.08º5o'W) (Fig. 1). It is an area of 180 km2, of which 62% is wetlands with a complex morphology. It is a mes- otidal coastal-plain lagoon-type estuary well mixed for normal river flow conditions, although high dis- charge in some winter months may cause moderate stratification in parts of the estuary (caeiro et al.,2005a; Ferreira et al., 2003). Most of the estuary is classified as a nature reserve and it is also a ramsar site due to the high biodiversity values. sado estuary is subjected to intensive land use practices, which play an important role in the local and national economy.
IntroDUctIonestuaries are areas of high productivity, crucial in the life history of many fish, invertebrates and birds. the sustainability of estuarine biodiversity is vitalto the ecological health of coastal regions. estuaries also rank among the most anthropogenic ecosystems on earth and are subjected to intensive environmental pressures. In particular, sediments can act as a sink and cycling centre for metallic contaminants, and therefore can be a potential source for metal bioac- cumulation by marine deposit and suspension feed- ing invertebrates, which may have adverse effects at complex levels of biological organization (lee et al., 2000). one major concern with the chemicals associated with sediments is that many commercial species, and their preys, are particularly vulnerable to toxic compounds given their close contact with sediment particles and interstitial water for extended periods of their life cycle. this provides a pathway for these chemicals to be transferred directly from sediments to organisms (Wright and Mason, 1999). Determining the ecological significance of trace metal contamination in sedimentary environments is difficult. Uptake and effects of sediment-associated contaminants are largely a function of bioavailabil- ity, which is strongly influenced by a set of physical, chemical and biological factors in the sediments. While most metals are naturally present in the aquatic environment, it is their presence at elevated concentrations that is a potential threat to aquatic life (rainbow et al., 1990).It is important, therefore, to determine whether ecologically keystone species of our estuaries are at risk due to toxic contaminants or whether different local populations are tolerant to bioavailable levels of toxicants that are potentially lethal elsewhere. In Sado estuary M. sanguinea is a herbivorous and sur- face detritivorous feeder with moderate or discreet surface mobility. It inhabits the intertidal mudflats of estuaries and coastal zones. It is broadly distributed in sado estuary (Portugal) wetlands, where it lives in deep borrows, and is particularly abundant in oldoyster production areas. It is among the key species in sado estuary, and functions as a major constitu- ent of the benthic biomass of mudflats as well as an important food item for crustaceans, fishes and waders (castro, 1993). M. sanguinea is also com- monly used as fresh bait and harvesting it is one of the most important socio-economic resources for local fishermen.the purpose of this study is to evaluate the influ- ence of weight on the bioaccumulation of sediment- bound Fe, Zn, cu, Pb and cd in M. sanguinea in Águas de Moura channel, located in central sado estuary.
MaterIal anD MetHoDsStudy areasado estuary is located on the southwest coast of Portugal (37º25'-38º40'n, 0.07º40'-0.08º5o'W) (Fig. 1). It is an area of 180 km2, of which 62% is wetlands with a complex morphology. It is a mes- otidal coastal-plain lagoon-type estuary well mixed for normal river flow conditions, although high dis- charge in some winter months may cause moderate stratification in parts of the estuary (caeiro et al.,2005a; Ferreira et al., 2003). Most of the estuary is classified as a nature reserve and it is also a ramsar site due to the high biodiversity values. sado estuary is subjected to intensive land use practices, which play an important role in the local and national economy.

Fig. 1. – sado estuary, south Portugal. letters and points indicate sampling sites: Z, Zambujal; a, arrábidas; G, Garças; P, Pinheiro
Table 1. – number of worms analyzed in sampling stations and in the 6 wet-weight classes (g)

the study site located in Águas de Moura chan- nel is quite shallow. It is intertidal with the largest salt marsh area of the estuary, where Marphysa sanguinea reaches high densities. It is also a high sa- linity area where hydrodynamic properties, nutrient dynamics and primary productivity patterns are very different from those in the adjacent areas (cabeçadas et al., 2000). Four sampling stations (Zambujal (Z), arrábidas (a), Garças (G) and Pinheiro (P)) were chosen according to the density of M. sanguinea, and avoiding intense harvesting sites.
Methodology
sediment and worms were collected at low tide in april 2002 at each station. sediment tripli- cate samples were collected with a previously acid washed cylindrical plastic tube (15 cm in diameter) placed directly on the gallery of the polychaete to a depth of 30 cm, immediately sealed then transported to the laboratory and stored at –80ºc before chemi- cal analysis. In the study area, the first 15 cm of the sediment samples were light-coloured and no H2s smell was noticed during collection, which indicates an oxygenated top sediment layer. the collected worms were carefully washed with seawater from the collection site to eliminate sediment and other particles. In the laboratory, only the top fraction (5 cm) of sediment was analyzed, and collected worms were divided into six weight classes (table 1) and placed in polyethylene covered tanks that had been previously acid washed. the bottom of the tanks was continuously aerated and filled with a fine layer of treated calcined sand and 5 m3 of filtered water. the sand was previously screened through a 0.5 mm sieve in order to remove algae and any associated macrofauna. afterwards, it was sterilized in an auto- clave (during 20 minutes at 1 atm) and then placed in a stove at 90ºc during 24 h. the worms were kept starved for 48 h in order to purge the gut contents and adhering sediment prior to metal analysis (Diéz et al., 2000). Water from sado estuary was changed daily, damaged individuals were removed and the faeces in each of the weight classes were collected for subsequent analysis. trace metals were determined by atomic absorption spectrophotometry (aas) ac- cording to the procedure developed by rantala and loring (1977) for sediments and faeces, and Vale and cortesão (1988) for tissues, using the multiple standard addition method.analyses of Pb and cd in all samples and cu in tissues were carried out with a Perkin elmer aanalyst100 atomic absorption spectrophotometer equipped with a deuterium background corrector. Pyrolytically coated furnace tubes were used. the flame technique (a conventional air/acetylene flame - Perkin elmer aanalyst 100 equipped with background corrector) was used to analyze al, Fe and Zn in all samples and cu in sediments and faeces. al was only determined in sediment in order to remove the grain size effect associated with the natural inputs of the sedimenta- tion process, which evidence the level of anthropo- genic contribution (loring, 1991; langston, et al.,1999; Villares, et al., 2003). Procedural blanks were prepared and analyzed with the samples. Interna- tional certified standards for sediments and faeces (Mess-1, Mess-2, Bcss-1 and 1646-a) and for tissues (DorM-1, DorM-2 and nBs-bovine liver) were used to control the accuracy of the procedures (tables 2 and 3).statistical analysis was performed using sPss (Statistical Package for the Social Sciences) soft- ware (Version 14; sPss Inc, chicago, Il). the relationships between metal concentrations and al content in sediment were examined using spear- man's rank correlation. concentrations in sampling sites (sediment and worm tissues) and metal con- centration in each wet-weight class were compared using the Kruskal-Wallis one-way anoVa fol- lowed by non-parametric multiple comparisons(lsD). a significance level of α = 0.05 was chosen. the effect of body weight on bioaccumulationwas first evaluated by multidimensional scaling.
Methodology
sediment and worms were collected at low tide in april 2002 at each station. sediment tripli- cate samples were collected with a previously acid washed cylindrical plastic tube (15 cm in diameter) placed directly on the gallery of the polychaete to a depth of 30 cm, immediately sealed then transported to the laboratory and stored at –80ºc before chemi- cal analysis. In the study area, the first 15 cm of the sediment samples were light-coloured and no H2s smell was noticed during collection, which indicates an oxygenated top sediment layer. the collected worms were carefully washed with seawater from the collection site to eliminate sediment and other particles. In the laboratory, only the top fraction (5 cm) of sediment was analyzed, and collected worms were divided into six weight classes (table 1) and placed in polyethylene covered tanks that had been previously acid washed. the bottom of the tanks was continuously aerated and filled with a fine layer of treated calcined sand and 5 m3 of filtered water. the sand was previously screened through a 0.5 mm sieve in order to remove algae and any associated macrofauna. afterwards, it was sterilized in an auto- clave (during 20 minutes at 1 atm) and then placed in a stove at 90ºc during 24 h. the worms were kept starved for 48 h in order to purge the gut contents and adhering sediment prior to metal analysis (Diéz et al., 2000). Water from sado estuary was changed daily, damaged individuals were removed and the faeces in each of the weight classes were collected for subsequent analysis. trace metals were determined by atomic absorption spectrophotometry (aas) ac- cording to the procedure developed by rantala and loring (1977) for sediments and faeces, and Vale and cortesão (1988) for tissues, using the multiple standard addition method.analyses of Pb and cd in all samples and cu in tissues were carried out with a Perkin elmer aanalyst100 atomic absorption spectrophotometer equipped with a deuterium background corrector. Pyrolytically coated furnace tubes were used. the flame technique (a conventional air/acetylene flame - Perkin elmer aanalyst 100 equipped with background corrector) was used to analyze al, Fe and Zn in all samples and cu in sediments and faeces. al was only determined in sediment in order to remove the grain size effect associated with the natural inputs of the sedimenta- tion process, which evidence the level of anthropo- genic contribution (loring, 1991; langston, et al.,1999; Villares, et al., 2003). Procedural blanks were prepared and analyzed with the samples. Interna- tional certified standards for sediments and faeces (Mess-1, Mess-2, Bcss-1 and 1646-a) and for tissues (DorM-1, DorM-2 and nBs-bovine liver) were used to control the accuracy of the procedures (tables 2 and 3).statistical analysis was performed using sPss (Statistical Package for the Social Sciences) soft- ware (Version 14; sPss Inc, chicago, Il). the relationships between metal concentrations and al content in sediment were examined using spear- man's rank correlation. concentrations in sampling sites (sediment and worm tissues) and metal con- centration in each wet-weight class were compared using the Kruskal-Wallis one-way anoVa fol- lowed by non-parametric multiple comparisons(lsD). a significance level of α = 0.05 was chosen. the effect of body weight on bioaccumulationwas first evaluated by multidimensional scaling.

the minimum number of dimensions necessary toreproduce the similarities/disparities between the wet-weight classes was evaluated according to the scree-plot criterion and by graphic analysis of the proximities transformed vs. distances. this analy- sis was refined by a non-hierarchical cluster analy- sis (K-means). the r-squared method was used as the decision criterion for the number of clusters to retain. the more important metals in the retained clusters were identified by the distance between the centre of the clusters and a statistical cluster F- anova analysis. the error probabilities associated with cluster results was evaluated with a two-wadiscriminant analysis (Wilks' lambda (∧) and theMahalanobis distance (DM2)). the variance-cov-ariance normality and homogeneity of each group were tested with shapiro-Wilk and M-Box tests re- spectively. a collinearity analysis was carried out in order to reinforce theresults obtained.
resUltsMetals in sedimentthe relationships between concentrations of pairs of metals in sediments (table 4) show that only Fe and Pb concentrations are significantly (p<0.01)>
resUltsMetals in sedimentthe relationships between concentrations of pairs of metals in sediments (table 4) show that only Fe and Pb concentrations are significantly (p<0.01)>

Fig. 2. – Metal concentrations ([al], [Fe] % and [Zn], [cu], [cd] µg g-1 dry weight ± s.d.) in the top 5 cm of sediment (n =3 samplings at each station) in Águas de Moura channel. s.d.- standard deviation. (a) normalized metal concentrations to al; (B) non-normalized metal concentrations. sediment quality guidelines for Florida coastal waters (MacDonald et al., 1996): _ _ _ t.e.l. (threshold effects level); P.e.l. (probable effects level)
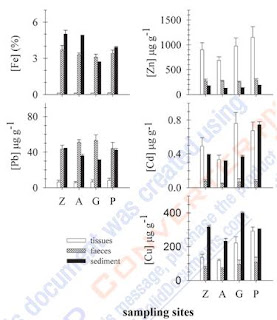
Fig. 3. – relationships between average concentrations of Fe, Zn, cu. Pb and cd (µg g-1 dry weight) in sediment (n =12), worm tissues and faeces (Z =780 w.; a =680 w.; G =660 w.; P =660 w.) in sampling stations. w - worms cu, and cd in sites near the channel mouth. ana- lyzed metals can be characterized into two groups with different behaviours: Fe and Pb are excreted and Zn, cu and cd are accumulated. In spite of the high excretion of Fe and Pb, these metals are still highly concentrated in tissues (table 5). Zn, cu and cd are definitely accumulated. However, the cu behaviour suggests that there is a degree of adaptation, which and it is not surprising in view of the very high concentrations found in sediments to which this population is exposed. Greater dif- ferences between sampling sites occurred in station Garças (G) where Fe concentrations in sediments were low (Z =5%; a =4.9%; G =2.7%; P =4%)(Fig. 4). Zn and cd have the largest bioaccumula- tion factor (BaF - ratio between metal concentra- tion in tissue and sediment [Met]/[Mes]) in site G p<0.05) i ="3e-2;" rsq ="0.99).">1 for all metals). cluster 8 (P2, P3 and P4) also shows positive values≤1, as well as clusters 6 (G1), 10 (G4) and 1 (Z1). the F-anoVa also shows that Zn (F =23.9) and cd(F =21) are the metals with most influence on clus- ter discrimination. However, F tests should be used only for descriptive purposes because clusters have been chosen to maximize differences among classes in different clusters. For this reason the error prob- abilities associated with these results were evaluated through a two discriminant analysis, and the exist- ence of collinear relationships was tested. all metals show normal distributions (p>0.05) for all classes, which is also confirmed by the variance-covariance homogeneity (table 7). In the first method (Wilks' lambda), although the results show that only Pb has significantly discriminant power (p =0.001), the step- wise analysis extracted two functions retaining Fe
Table 5. – Metal concentration (µg g-1 d.w.) averaged over the whole populations and standard deviation (±sd) in Marphysa sanguinea (Ms) compared with metal concentrations in arenicola marina (aM); nereis diversicolor (nD); nereis virens (nV); heteromastus filiformes (HF) and eurythoe complanata (ec) in several polluted estuary environments. the 95% confidence intervals are shown in brackets; ♦ (% value); nd, not determined.


Fig. 4. – Bioaccumulation factor (ratio between metal concentra- tion in tissue [Me]t and sediment [Me]s- BaF) in the M. sanguinea population in Águas de Moura channel.
Table 6. – Wet-weight class classification for the method K-means with K = 11 clusters and one-way anoVa for each metal. the weight class corresponding to the clusters is shown in brackets.


Fig. 5. – averaged metal concentrations (%±s.d. (Fe) and µg g-1 d.w.±s.d. (Pb, Zn, cu and cd) in each wet-weight class and in whole worm tissues (a) and faeces (B) along Águas de Moura channel. s.d. - standard deviation; c1 [0.5-1]g; c2 [1-1.5]g; c3[1.5-2]g; c4[2-2.5]g; c5[2.5-3]g; c6[3-3.5]g wet-weight; nc1 =234; nc2 =550; nc3 =731; nc4 =650; nc5 =407; nc6 =208
as statistically significant, although the discriminant power is questionable (p =0.06). Function 1, defined essentially by Pb, explains 76% of the variability and discriminates all the wet-weight classes signifi-cantly (∧ =0.20; χ2(10) =30.57; p =0.001). However,the second retained function, defined by Fe, does not discriminate all the classes significantly (∧ =0.61; χ2(4) =9.44; p =0.05). In order to assure that the se- lected metals (Pb and Fe) are in fact important, a new analysis was carried out using DM2. In this method
as statistically significant, although the discriminant power is questionable (p =0.06). Function 1, defined essentially by Pb, explains 76% of the variability and discriminates all the wet-weight classes signifi-cantly (∧ =0.20; χ2(10) =30.57; p =0.001). However,the second retained function, defined by Fe, does not discriminate all the classes significantly (∧ =0.61; χ2(4) =9.44; p =0.05). In order to assure that the se- lected metals (Pb and Fe) are in fact important, a new analysis was carried out using DM2. In this method

only Pb was selected and the function extracted discriminates all the weight classes significantly (∧=0.353; χ2(5) =20.9; p =0.001). table 8 presents theclassification statistics of wet-weight classes withthe respective classification functions generated by these two analyses. In the first one, 45.8% of the classes were correctly classified, against 33.3% in the second one. comparing the two methods, we can see that Pb without the influence of Fe, in spite of its weak performance, classified the two small classes very well (c1 and c2 - 75% for both). When the two metals are associated they perform better for the largest classes (c4 to c6). the smallest class (c1) was always well classified with the two methods
Table 8. – original classification results used in the discriminant analysis with the two methods: Wilks' lambda (∧) and the Mahalanobis distance. WWc, wet-weight classes.

the results obtained with the Kruskal-Wallis analy- sis (table 9) confirm the results achieved for Pb and also show that Zn is an important element in weight class differentiation.
Table 9. – Kruskal Wallis test between metal concentrations in each wet-weight class; Grouping Variable: classes.

DIscUssIon
Metals in sediment
Metal concentrations in sediment were gener- ally higher at the entrance of the channel as has been previously described by cortesão (2003). the high correlations obtained between Fe/al and Pb/al suggest that Fe and Pb are closely associated with aluminosilicates. Moreover, inside this fine fraction the close relationship between Fe and Pb shows that Pb is mainly associated with Fe oxyhydroxides (FeooH), which also have a great capacity to re- tain trace metals like cd and cu. In fact the larg- est differences between stations occurred where Feconcentrations were low. the [Me]/al ratio for Zn and cd increased from upstream to downstreamalong Águas de Moura channel, which reflects the influence of anthropogenic sources. Higher levels of cu, Zn and cd occurred at downstream stations (G and P), evidencing the importance of sado river as a metal source for the estuary (cortesão, 2003; caeiro et al., 2005b).considering the potential toxicity of sediments in the study area, reference values given by MacDon- ald et al. (1996) suggest that there is a physiologi- cal cu-adaptation in Marphysa sanguinea. In fact all analyzed specimens were in apparently healthy condition considering animal activity and the game- togenic development in most of them. High cu-lev- els (200-2000 µg g-1 dry weight) in the sediment are known to be toxic to aquatic animals including meio- and macrofauna (Morrisey et al., 1996; austen and somerfield, 1997).
Metals in worm tissues
comparing the mean metal-concentration in the M. sanguinea population in the studied channel with the concentrations in other polychaete species from other estuarine environments, the present values can be considered relatively high for Fe, Zn, cu and Pb, and moderate for cd. the results obtained for Fe and Pb could be explained by low availability. Most of the Fe is in an unavailable form, as Fe-hydroxides or sulphides, but it seems that a substantial amount is still available to be absorbed (table 5), which is sug- gested by the high Fe excretion superior to sediment levels (1.4±0.09) in station G. the high Pb excre- tions, superior to Pb sediment levels in all stations (1.3±0.2), suggest that although there is very low availability, there is some accumulation and even- tually other sources of Pb intake. the significant correlations between Fe and Pb also suggest that the Fe concentration in sediment may influence the availability of Pb by influencing the physicochemi- cal form of sediment-bound Pb. luoma and Bryan (1978) found that Fe influences Pb availability in Scrobicularia plana. In addition, the significant dif- ferences that occurred between station G, where Fe concentrations in sediments were low, and the other stations, suggest that Fe influences the availability of the other metals.It is well known that Zn, cu and cd are accumu- lated in hediste (syn. nereis) diversicolor and other annelids in agreement with the metal concentrations in the sediment (Berthet et al., 2003; nipper and carr,2003). the degree to which these metals accumulate in M. sanguinea varied considerably. Zn showed the highest accumulation. at all sampling stations, Zn concentrations in M. sanguinea exceeded the con- centrations in the sediments (5.8±1.4), with an aver- age BaF= 6±1.2, which suggests sequestration or other uptake sources. For example, in station G the tissue concentration reached up to seven times the sediment values. Zn concentrations found here (560 to 1502 µg Zn g-1) were much higher in comparison to those presented in the cited literature. In contrast, cd levels in M. sanguinea approaches those in the sediments at all surveyed sites except at station G (1.6±0.3), with an average BaF of 1.3±0.5. the low excretion obviously suggests accumulation. cd val- ues obtained in this work are comparable with those of n. diversicolor in table 5. In light of the very high concentrations in sediments referred to previ- ously and the accumulation under certain limits, the results for cu show the existence of an obvious adaptation. In fact, the values obtained in this study (102 to 320 µg cu g-1) are relatively high in com- parison with those presented in table 5. these very high cu levels, especially in sediments, suggest that there is some kind of regulation. However, bioac- cumulation is the result of a complex interactivity between sediment characteristics and animal physi- ology and therefore factors such as animal size need to be considered.
Influence of body weight on metal accumulation and excretion
the bioaccumulation results obtained for Mar- physa populations along Águas de Moura channel previously discussed, indicate that Zn, cu and cd bioaccumulation does not seem to be supported when body weight is considered. In fact, cu and cd concentrations are not higher in bigger specimens while Pb, Zn and Fe concentrations tend to have a negative relationship with body size. Is there size- dependence for all metals including cu and cd? the observed decline in some metal body burdens in the bigger worms could be a result of growth-dilution. In fact, this polychaete is considerably large and has a short life cycle (1.4 years) (castro, 1993), and it is very probable that the metal concentration in tis- sues is influenced by its fast growth. Previous works on growth rates of polychaetes, especially in n. diversicolor (Fidalgo e costa 2001), nereis virens (olive et al., 1991), arenicola marina (De Wilde and Berghuis, 1979; Farke and Berghuis, 1979) and M. sanguinea (castro, 1993) revealed a significantlyhigher rate in the first months. according to ahrens et al. (2001), the prolonged resident time of ingested food in juveniles of nereis succinea, allied with their digestive chemistry, facilitates desorption and subse- quently the increased uptake of sediment-bound con- taminants. However, assuming that most metals are sequestered in hard structures and epithelial surfaces (such as jaws, cuticle and the gut lining) then bound- metals would increase at a slower rate than weight (which scales with an exponent of x3) compared to surfaces (x2). this could explain why bigger worms have relatively less metals in their tissues, i.e. it is mostly in hard parts, which are associated with total surface area. the very high Zn levels provide evi- dence of sequestration within the animals. Bryan and Gibbs (1979) proposed that the jaws might serve as a metal–sink, sequestering toxic levels of Zn absorbed from the sediment away from the living tissue (1.5% of the dry weight and 70-80% of the total metal con- tent in nereis jaws). they also demonstrated that Zn concentrations fall very significantly with increasing size, and significant concentrations of other metals, including Fe, are also present. Further observations (lichtenegger et al., 2003, Broomell et al., 2006,2007) demonstrated that Zn levels in nereis jaws were high, regardless of the environmental context, which led to the hypothesis that metals might con- tribute to their mechanical properties.a complex jaw apparatus consisting of ventral mandibles and dorsal maxillae is characteristic of polychaetes of the eunicidae family. the high levels of Zn and Pb in the smallest wet-weight classes of M. sanguinea can be related to the carbonate nature of the jaws (structures composed of calcium carbonate and/or scleroproteins (Paxton, 2006; Voss-Foucart et al., 1973).the results obtained show that Fe influences large weight classes. the relatively high level of Fe in small worms possibly reflects the deposition of Fe-oxides on their exposed surfaces. the source of this Fe may be the overlying water or, more prob- ably, the interstitial water found in the reduced sub- surface sediments into which the irrigated burrows penetrate. the high Fe levels in the youngest M. sanguinea worms could also be the result of meta- bolic needs in view of the essential role of Fe in, for example, Marphysa haemoglobin. the absence of a clear relationship between cu and cd concentrations and body weight can be explained by physiological factors like reproductive maturation (Howard and Brown, 1983), copper regulation capacity (Méndez and Páez-osuna, 1998), and specific metabolic needs. However, we found size-dependence for all the metals, including cu and cd (even though the r2 are lower).In conclusion, the results obtained suggest: (1) Fe has a strong influence on metal availability, mainly for Pb; (2) M. sanguinea is adapted to the high cu and Fe levels in Àguas de Moura channel; (3) the significant differences in Zn and Pb concentrations between the small weight classes (c1 and c2) and larger ones (c3 to c6) indicate that it is important to consider the worm's weight in environmental moni- toring programmes. However, further investiga- tions addressing other ecotoxicological aspects (e.g. metabolic routes involved in metal accumulation and excretion) are encouraged in order to confirm this suggestion and to reinforce the relevance of this species to be included in environmental monitoring programmes in sado estuary.
IBAÑEZ JESUS
EES
No hay comentarios:
Publicar un comentario